ChemistryWiki | Applications For Redox Rxn - Metal Plating | RecentChanges | Preferences
Changing Chemical Energy into Electrical Energy (and visa versa)
As you know, Redox rxn (looking at oxidation numbers) show the movement of electrons as the chemical reaction occurs. This movement requires energy to occur. Where does the energy come from to movement the electrons? Answer: It comes from difference in potential energy in the chemical species of the reactant compared to potential energy of these chemical species as products. So as the reaction occurs, there is a change in this chemical energy difference, "chemical potential", until you run out of reactants and the reaction stops (in Redox rxn, the movement of electron stops).
This "chemical potential" not only exists in compounds but also between elements. This is reason why one metal that is higher up on the "strength chart" than another metal will allow the single replacement reaction to occur. As with the single replacement reaction (and all reactions), if the "chemical potential" in the reactant is not greater than the chemical potential of the products, the reaction does not occur (except when it gets outside help!!)
So, to summarize, two major things you need to know:
1)Reactions occur because the "chemical potential" of reactant is greater than the products (flowing of electrons in Redo rxn
2)The reaction will continue (electrons will flow) until the chemical potential difference ends.
There is two main applications from this knowledge relating to Redox rxns.
1)Chemical energy (potential) to Electrical energy (electron flow) - Electrochemical Cell (Batteries)
2)Electrical energy to Chemical energy - Electroplating/electrolysis (Plating metal for jewelry)
Electrochemical Cell (Daniel or Voltaic Cell)
In this application we are changing chemical energy (potential) into electrical energy. If you separate the species (both reactant & product) that are reducing (i.e. reduction half-cell) from the species that are being oxidized (i.e. oxidation half-cell), you can direct the electrons that are moving to places that can operate electrical device. This is the general principle of a battery. The figure below shows you an electrochemical cell. Modern batteries use all solid phase species not like the solution phases you see in this figure.
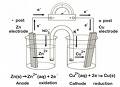
Click here to go to a webpage to do an online electrochemical cell, Online Lab for Electrochemical Cell
Electroplating
In this application we are changing electrical energy into chemical energy (potential). Since you are providing outside "help" (in form of energy) to the chemical reaction, you can have reactions that would not occur on their own (called spontaneous). This is how a thin layer of metal (i.e. gold, silver, copper, chromium, nickel) are deposited (called plated) onto a base metal (usually stronger & cheaper). The longer you apply the electrical energy, the more metal you plate onto the base metal.
