ChemistryWiki | RecentChanges | Preferences
Part I
For the following problems, please show the answer with 1 more sig fig or 1 more decimal place with units (and if need to be Scientific notation) and then the answer in the correct number of sig fig with units (and if needed in Scientific notation).
1) (0.201m) (15.001m) (9.3m) = __________________ = ____________________
2) ( 3.21E-4 m) ( 6.21E9m) = ____________________ = _____________________
3) 13.210 L - 0.753 L = __________________ = ____________________
4) 4.28E7g / 2.13E-3 sec = _________________ = _____________________
5. (8.03E-5sec) (3.72E-2sec) = ___________________ = _______________________
Part II
Please write in the space provide the number of sig fig in the number. Also indicate if any zero are significant (i.e. sandwich - sig, before not sig, etc)
1. 8765 m ________________________
2. 0.000073m ______________________
3. 40.07 g _________________________
4. 321 L _________________________
5. 20401 L ______________________
6. 6.1200 L ______________________
7. 0.070030 m ____________________
8. 3000 L _______________________
9. 0.078 g _______________________
10. 8011 g ______________________
Part III
Provide the scientific notation for the following numbers. Include correct number of significant figures (assume all trailing are not significant).
1. 0.0000205 m ___________________________
2. 410300 L _____________________________
3. 0.78102 g ________________________
4. 8200000 mg ______________________
5. 623100 mL _______________________
Part III
Answer the following problems using Dimensional Analysis. Show all work as stated in notes and homework.
1. How many mL in 3.5 kL?
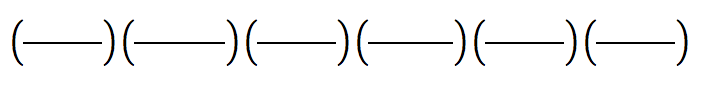 = __________________ = __________________
= __________________ = __________________
2. How many mg in 4.14 lbs.
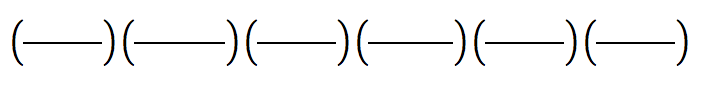 = __________________ = __________________
= __________________ = __________________
3. How fast ( in cm/s) is Principle Donovan go if he is driving at 53.1 mile/hr (mph).
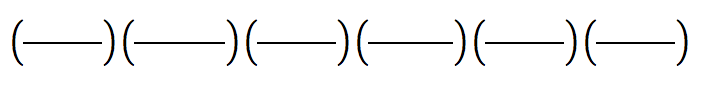 = __________________ = __________________
= __________________ = __________________
4. What is the density of a substance that has a volume of 32.7 mL and a mass of 124.1g ?
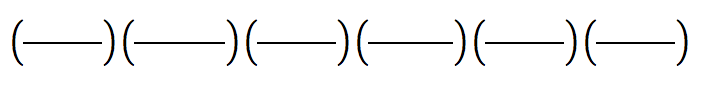 = __________________ = __________________
= __________________ = __________________
5. What is the density of the solid that has a mass of 23.5g and the volume was found the water displacement. The volume of the water before adding the solid was 15.32mL and after adding the solid, the resulting volume is 26.47mL.
 = __________________ = __________________
= __________________ = __________________ = __________________ = __________________
= __________________ = __________________ = __________________ = __________________
= __________________ = __________________ = __________________ = __________________
= __________________ = __________________