ChemistryWiki | RecentChanges | Preferences
Colligative Properties - Solvent vs. Solution Properties
A solvent has a specific set of physical properties. Once you add in solute to create a solution, the physical properties of the solvent has changed. Therefore, the properties of the solution are different from the solvent. The properties that show these differents are the Colligative Properties where the two properties we will be dealing with are freezing point and boiling point.
Boiling Point Elevation
To boil water, you must add energy to the system to overcome the intermolecular forces (IMF) between the water molecules to enable the molecules to go into the gas phase (really called vapor, here). If you add in a solute (when you made a solution), you will now need to add more energy to the system ( increase the boiling point) to boil the same amount of water. This occurrence can be explain in several ways. One, in a solution, some of the water molecules that use to be on the surface of the water are now replaced with solute that do not go into the vapor phase. Second, the solute you added to the solvent provide additional IMF to the system (i.e. adding an ionic compound) so that more energy is required to overcome this addition to force for the water molecules to enter the vapor phase.
Therefore, the more solute added, the higher difference between the boiling point of the solution compared to the boiling point of just the solvent. This difference is called the Boiling Point Elevation. The equation for the Boiling Point Elevation (deltaT) is the boiling point of the solution - boiling point of the solvent or : deltaT = Tsolution - Tsolvent.
This difference is a function of two parameters: 1) how much solute you put into the solvent measured in molality (m) and 2) a property of the solvent itself, called the Boiling Point Elevation Constant (Kb), that you find in your book on Pg.457 Table 16.21. The equation is deltaT = (Kb)*(m) where Kb has units of degC / m.
Therefore, the equations are deltaT = Tsolution - Tsolvent and deltaT = (Kb)*(m)
Freezing Point Depression
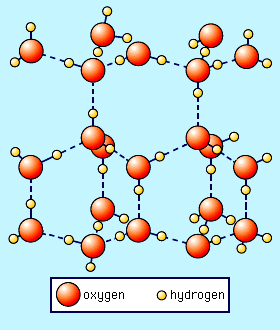
A similar discussion as the Boiling Point Elevation can be made for the Freezing Point Depression. The physical difference here is that we are talking about freezing of water (see figure above) where the water molecules link up in a hexagonal crystal arrangement (called ice) where one water molecules orients itself with respect to another water molecules (the dotted line in figure indicate hydrogen bonding not an actual chemical bond). However, when solutes are added to the water, these solute molecules get in the way of the water molecules linking together to form the hexagonal arrangement so you need to slow all the molecules down (decreasing the temperature) so that the water molecules can move the solute molecules away so to linkup. Therefore, the solvent freezing point will be higher than the solution's freezing point. The difference is called the freezing point depression and has the equation:
deltaT = Tsolvent - Tsolution
The freezing point depression is a function of the 1) how much solute you put into the solvent measured in molality (m) and 2) a property of the solvent itself, called the Freezing Point Depression constant (Kf) that you can find in your book on Pg.455 Table 16.20.
The equation is deltaT = (Kf)*(m) where Kf has units of degC / m.
Therefore, the equations are deltaT = Tsolvent - Tsolution and deltaT = (Kf)*(m)
For calculating purposes, the freezing point depression and boiling point elevation problems are done basically the same way expect for how deltaT is found and that you use different constant (Kb vs. Kf). We will show an example using the boiling point elevation
Boiling Point Elevation Example
What is the boiling point of a solution where 9.56g of calcium chloride is added to 455grams of water? The boiling point elevation constant, Kb, is 1.86 degC / m.
The equation to find deltaT is: DeltaT?= (Kb)*(m)
m = moles CaCl2 / kgH2O
where the molar mass is 1(40g) + 2(35.5g) = 111g/mole
mole = (9.56g)*(mole / 111g) = 0.08612moles CaCl2
m = 0.08612moles CaCl2 / 0.455kg H2O = 0.1892m
deltaT = (1.86degC / m)* (0.1892m) = 0.3519 deg C
deltaT = Tsolution - Tsolvent ===> Tsolution = deltaT + Tsolvent
Tsolution = 0.3519degC + 100.degC = 100.35degC, the Boiling point of solution (yes, I know I broke sig fig rules here !!!!!)
