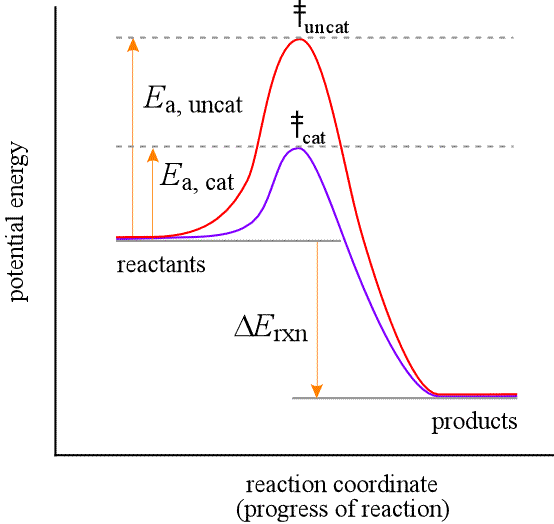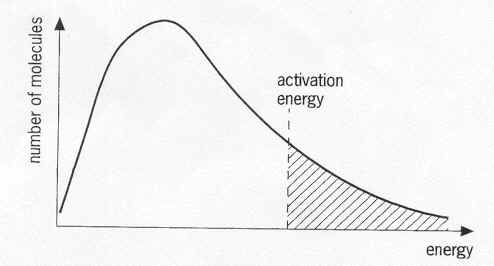
b. Temperature increase/change (means giving every particle in the substance more energy, equally).
a. The nature of the reactants
How the substance is bonded together plays a part. For example, ionic compounds tend to react faster since once in solution they are actual ions that rearrange very quickly. On the other hand, reaction with covalently bonded elements require their chemical bonds to be broken and then the electrons are reoriented (i.e. all following Rule of Eight) with new atoms to form new compounds etc. Therefore, this reaction with be slower requiring more time for products to be made.

b. Temperature increase/change (means giving every particle in the substance more energy, equally).
c. Amount of Reactant near each other per volume: (so have more overall number of particles, at same energy profile)