ChemistryWiki | RecentChanges | Preferences
Showing revision 3
Show the work explained in class to determine the answers to the following problems (answers are in link to this webpage):
1 mile = 1.61 km , 12 in = 1 ft , 1cm3 = 1 mL 3.785 L = 1 gal 454g = 1lb
1) A substance has a mass of 435 g and occupies a volume of 639 ml. What is its density in g/ml?
2) A substance occupies 204mL and has a mass of 136g, what is its density in g/ml?
3) A substance has a density of 3.04 g/ml, how many grams (g) does it weigh if the substance has a volume of 425mL?
4) What is the volume of a solid ( in ml) that has a mass of 1572.g if the density is 0.291 g/cm3 (Hint look at above conversion factors)?
3) A substance has a density of 0.410 g/ml, how many mg does it weigh if the substance has a volume of 2.1L?
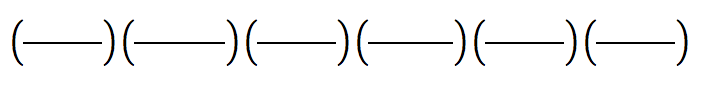 = __________________ = __________________
= __________________ = __________________
4) A substance has a density of 12.1 g/ml, how many lbs does it weigh if the substance has a volume of 7.42L?
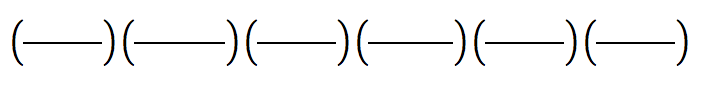 = __________________ = __________________
= __________________ = __________________
5) What is the volume of a solid ( in ml ) that has a mass of 0.621kg if the density is 2.17 g/L ?
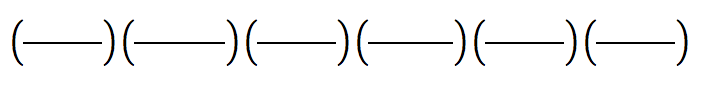 = __________________ = __________________
= __________________ = __________________
6) Which substances from the problems above (use the question # as reference) would float on water and why? (Hint density of water is 1.00g/ml or 1.00g/cm3 or 1000.g/L)
Answer:
1) 1.29 kg
2) 1.43 gal
3) 8.61E5 gal
4) 198 lb
5) 2.86E5 ml
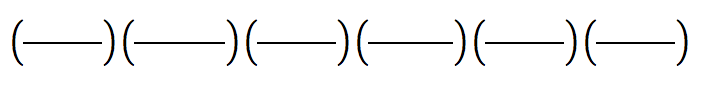 = __________________ = __________________
= __________________ = __________________ = __________________ = __________________
= __________________ = __________________ = __________________ = __________________
= __________________ = __________________