ChemistryWiki | RecentChanges | Preferences
Showing revision 8
Two Pillars of Chemical Reactions : Kinetic and Thermodynamics
In a chemical reaction, there are three questions that are commonly asked?
1. Does this chemical reaction occur?
2. How fast is the chemical reaction or how fast are the products formed (called rate of reaction)?
3. How do the reactants rearrange to make the product (called reaction mechanism)
Thermodynamics is the study of the movement (-dynamic) of heat/energy (thermo-) in chemical reaction. Almost all chemical reaction have either a total gain of heat/energy (or total loss of heat/energy) as the chemical reaction proceeds. This change in heat/energy determines if the chemical reaction occurs or not. Therefore, Thermodynamics answers Question #1 or does the chemical reaction occurs or not. It does not. however, explain how fast or the reaction mechanism of the chemical reaction.
How fast and what is the reaction mechanism for a chemical reaction is the domain of Kinetics. Therefore, Kinetics answers Question #2 & #3 and will be the topic we will deal with now.
?Why do reactants form products? Smashmouth approach (called Collision Theory)
Collision Theory
Rate of the chemical reaction:
Units of Rate:
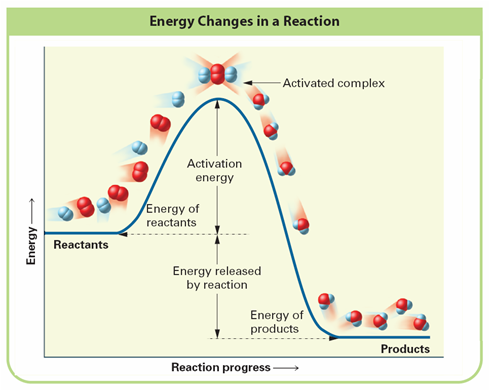
Activation Energy:
Activated Complex:
Do all collisions of reactants form products?
Effective Collisions
Two factors for an effective collision:
a.
b.
