ChemistryWiki | RecentChanges | Preferences
Difference (from revision 8 to current revision)
(minor diff, author diff)
(The revisions are identical or unavailable.)
Acidic, Basic, and Neutral Solutions
Table in webpage is from www.chem.ubc.ca)
The difference between acidic, basic, and neutral solution is solely a function of pH as the following table shows.
pH vs. Type of Solution |
| pH Range |
Type of Solution |
| pH<7.00 |
Acidic |
| pH=7.00 |
Neutral |
| pH>7.00 |
Basic |
The relative concentrations of the [H3O+] or [OH1-], pH and pOH values and type of solutions (acidic,basic & neutral) are highlighted in the following table (from .
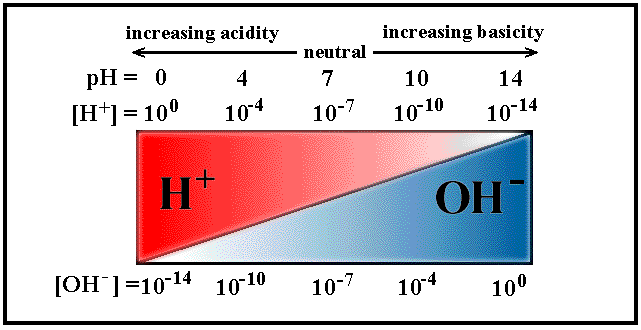
Tables on this webpage are from www.chem.ubc.ca

